Fluxed Up: The Fracture Healing of Ruby
By Richard W. Hughes
Palagems.com
With an Appendix by John L. Emmett
Note: This article received the first-place Richard T. Liddicoat Journalism Award from the American Gem Society in 2005. The late Richard T. Liddicoat is known as the Father of Modern Gemology. The Richard T. Liddicoat Journalism Awards honor journalists that have made exceptional contributions to the understanding of gemology, as well as AGS ideals of dedication to proven ethics, knowledge, and consumer protection within the jewelry industry.
Introduction
In 1997, a colleague and I submitted a paper to a gem industry trade association for publication in their newsletter. The article discussed a specialized heating technique used to treat over 95% of the ruby traded in the world market. To our great surprise, the editor rejected the article, deeming it “too controversial.”[1] In the words of the official, “many mainstream journalists read our publication and if they got hold of this story it could mean big trouble.”
Just what treatment could possibly be so nefarious that it could not even be discussed in the polite pages of an industry publication? I speak of the use of fluxes to “heal” open fractures in ruby.
Since that time, the process has been a point of discussion at dozens of meetings between both traders and gemologists. While some today have a solid knowledge of flux healing, it is surprising that so many traders and even gemologists do not grasp the treatment method and its impact on a gem. The following is designed to shine a bit of light into this dark industry corner.
History lesson
The use of fluxes during heat treatment is not a recent invention. In the early 1980s, while I was directing Bangkok’s Asian Institute of Gemological Sciences (AIGS), a number of Burmese rubies with unusual characteristics were brought into our lab for testing. Based on the inclusions, it was clear they originated from the Mogok mines. But unlike the classic Mogok stones, these rubies showed numerous thick wispy fingerprints. There was also evidence of high-temperature heat treatment.
Someone had apparently been cooking Mogok ruby. It was equally clear that the heat-treatment process was healing fractures, either pre-existing, or those produced by the stresses of the heat treatment itself.
For a brief period, we saw many such stones. Then, just as suddenly as they appeared, they were gone.
Healing
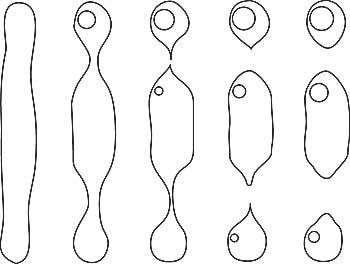
W.F. Eppler (1959) and Edwin Roedder (1962) were among the first to clearly describe how fingerprints form in gems, a process illustrated in Figure 1.
At any point after a crystal grows, it may fracture. Given the proper conditions, that fracture may later heal closed, leaving a scar-like inclusion typically known as a “fingerprint”
or “feather.”
 |
| Figure 1. Formation of a fingerprint The healing of a crack in a crystal, resulting in secondary cavities (‘fingerprint’). A. A fracture develops during or after the crystal’s growth. B. Healing begins. Growth solutions flow into the fracture and/or the inner walls of the crack are partially dissolved, beginning the healing process. C. Healing continues. Dissolved nutrients are re-deposited on the inner walls of the crack as the healing proceeds. D. Eventually the fluid-filled cavities become more angular in shape, turning into fluid-filled negative crystals arranged in a fingerprint pattern. The fluid that remains behind has been leeched of its nutrients. These pockets containing exhausted growth solutions are smaller along the inner edges and bigger near the outer edges of the original crack. (After Roedder, 1962) |
The healing process involves exposure to a combination of heat and solvents. In the ground, elevated temperatures and solvents produce healing of fractures via corundum-containing solutions. Dissolved nutrients (solute) may come from solvents dissolving surrounding crystals, the exterior of the crystal itself, or the interior walls of the fracture. This dissolved nutrient material then regrows on the walls of the crack, “healing” it closed. But an internal scar remains, something we term a “fingerprint” inclusion.
Eppler (1959) actually reproduced this process in the lab, producing fingerprint inclusions in Verneuil synthetic rubies by inserting fractured stones into hydrothermal autoclaves. Upon removal, the fractures had healed, resulting in fingerprint inclusions.
 |
| Figure 2. Anatomy of a healed fracture A well-healed fracture in a sapphire lying roughly parallel to the basal plane. The healed areas appear dark, while the undigested fluids are highly reflective. Note that the pattern of healing relates to the underlying crystal structure, with angles of healed areas following the underlying crystallographic structure (in this case, at 60/120°). Photo © Richard W. Hughes |
Fill ’er up—Surface repair (glass infilling)
A year later a group of rubies came into the AIGS lab with suspicious characteristics. In this case, large surface cavities had been filled with glass (Hughes, 1984). One of these stones is shown in Figure 3.
This process became known as “surface repair” or “glass-infilling.” Its purpose was to fill unsightly surface cavities, thus allowing larger stones to be cut.
The first of these stones had huge glass-filled cavities. When customers balked, the gems largely disappeared from the market. But the idea was now in the minds of both dealers and gemologists—glass might be used to fill surface cavities.
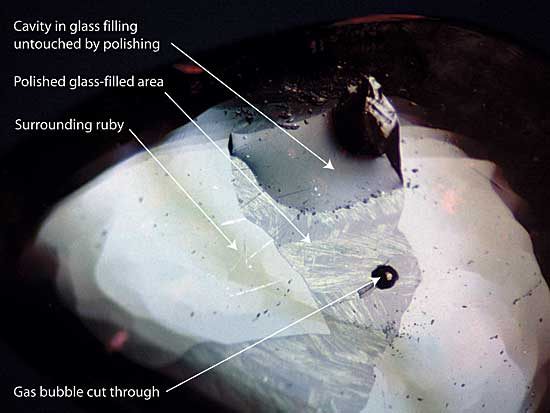 |
| Figure 3. Surface repair (glass infilling) A large glass-filled surface cavity is clearly visible in this treated Thai/Cambodian ruby cabochon. The glass has a lower luster than the surrounding ruby, and in places has started to devitrify (crystallize). The upper portion of the filling is below the surface of the gem and so was not touched by the polishing wheel. The circular black dot in the center is a cavity resulting from a gas bubble cut through during the cutting process. Photo © R.W. Hughes |
Desperately seeking glass
In the years that followed, gemologists were zealous in seeking out glass-filled surface cavities, often to the point of flagging tiny amounts resulting from inclusions that had melted during heat treatment, fillings that had absolutely no impact on the weight or appearance of a gem.
This situation left dealers scratching their heads. Stones with “accidental” glass fillings of no consequence were being lumped together with other stones featuring surface cavities deliberately filled with glass to add weight or hide naturals. Eventually at AIGS, we came to an arrangement. When we found accidental fillings, if the owner was agreeable we simply removed the glass with hydrofluoric acid, [2] thus allowing us to issue a cert without the nasty “glass filling” comments.
 |
| Figure 4. Mong Hsu ruby before and after heat treatment Mong Hsu ruby before (left) and after (right) heat treatment. This clearly shows that most Mong Hsu ruby is not a viable gem without heat treatment. Photo © R.W. Hughes |
As for the gems with deliberate glass fillings, owners wanted no part in removing them since gaping cavities would remain. Such stones were clearly flagged on ID reports and buyers rejected them. Thus it was not long before they mostly disappeared from the market.
Enter Mong Hsu
In the early 1990s, a major discovery of ruby was made in Burma’s Shan State, at Mong Hsu (pronounced ‘Maing Shu’ by the Shan; ‘Mong Shu’ by the Burmese), northeast of the provincial capital of Taunggyi. So important were these discoveries that
—within just a few years—Mong Hsu rubies constituted over 95% of the faceted ruby entering world markets. This remains true to this day.
But Mong Hsu is not Mogok. Before heat treatment, the Mong Hsu ruby is almost always an ugly duckling.
There are two major problems. The first is dense silk/particle clouds and a strong purplish color, making most stones look like low-grade, cloudy rhodolite garnet. This is mainly due to the crystal’s unusual blue cores. Ordinary heat-treatment removes the blue, as well as removing silk, making the final product a rich, clear red. The market generally accepts such heated stones without a quibble.
 |
| Figure 5. Diagram of a flux-healed fracture The mechanism of flux healing of a fracture in corundum. A. Open fracture/fissure, unhealed. B. During heat treatment, flux enters the fracture and dissolves the walls of the crack. C. During cooling, dissolved corundum recrystallizes in the crack, thus healing it closed. The newly crystallized ruby is essentially a synthetic ruby grown in the crack alone. It contains small pockets of now-solidified flux glass, along with some trapped gas pockets. For purposes of this diagram, the surrounding natural ruby and the synthetic ruby in the crack are shown in two different colors. In reality, no distinction can be seen between the surrounding ruby and the newly grown synthetic ruby. D. Any flux glass present on the surface can be dissolved away with acid. The synthetic ruby in the crack is unaffected by the acid, as is the ruby as a whole. (Illustration © R.W. Hughes; modified from Hänni, 2001, SSEF) |
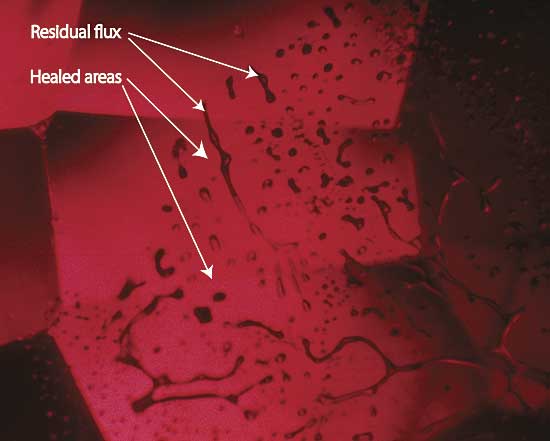 |
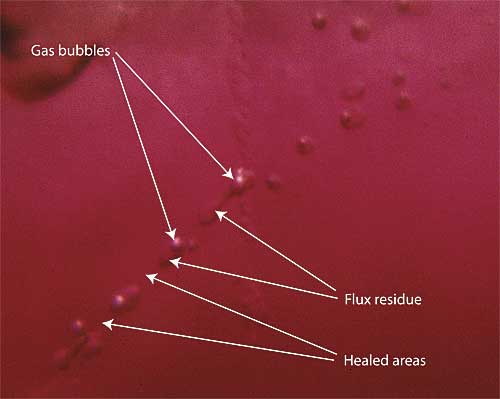
| Figure 6. Flux-healed fracture Moderate magnification reveals a flux-healed fracture in a Mong Hsu ruby from Burma. The irregular dark areas are pockets of residual flux, while the red areas in between are where the once open fracture has healed shut with microscopic amounts of what is essentially synthetic ruby. In some places, the flux residue appears transparent. This is an illusion produced by reflection off the surfaces of the flux pockets. Photo © R.W. Hughes |
Crack be gone
This is not the case for the second problem. Most Mong Hsu stones come out of the ground in a heavily fractured state. But Thai burners are nothing if not ingenious. For years some had used fluxes in conjunction with their burning. This pow chemie (‘heating with chemicals’) was supposedly done for a variety of reasons. According to some, fluxes produced a shine on the surface of the rough after cooking, making the color look better. Others said it helped create a desired furnace atmosphere. Some even believed that it helped prevent thermal shock during heating (an idea which has been discredited; see Emmett, 1999).
When I examined my first parcel of Mong Hsu ruby, the coin dropped. Those Mogok rubies with their twisted drippy fingerprints from so many years before were early examples of flux-assisted fracture healing. And with Mong Hsu rubies, burners were taking that treatment to the next level.
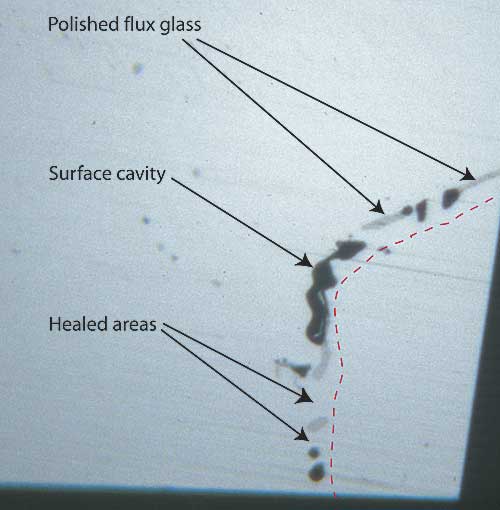 |
| Figure 7. Surface of a flux-healed fracture A highly magnified photo showing a single facet’s surface in reflected light where a fracture breaks the surface in a flux-healed Mong Hsu ruby. The dotted red line shows the path of what was once an open fracture, displaced slightly to the right so you can see surface detail. The irregular black areas are surface cavities where bubbles in the flux were cut through, while the irregular gray areas are residual flux glass that has been polished. Note the lower luster compared with the surrounding corundum. In between the surface cavities and flux glass are healed areas, indistinguishable from the surrounding corundum. Photo © R.W. Hughes |
Unfortunately, this information never quite filtered down to the dealer on the street. Small bits of glassy flux residue were often found on the surfaces of finished stones. Since the amounts involved were tiny, many assumed the glass to be an accidental by-product of heat treatment. The reality was far different—the tiny flux remnants were but droppings on the trail of a massive treatment beast—one the gem trade has yet to fully confront. It was this secret that the gem industry was so afraid to bring out in the open.
These are strong words, but carefully chosen. In the late 1990s, the emerald trade was rocked by non-disclosure of clarity enhancements. Incredibly, what has been done to ruby over the past decade is far more radical, and yet has completely slipped under the radar.
What the flux is this?
One major factor that separates fine gems from inferior is clarity. Heavily fractured stones are common in nature, but clean gems are decidedly not. If you can take a fractured gem and remove the fractures, you are radically skewing the equation that keeps the prices of fine natural gems high. What is being done today with Mong Hsu rubies is the removal of fractures.
How often is this treatment applied with respect to Mong Hsu rubies? So often that over the past decade I can recall seeing only a handful of stones from that deposit which had open fractures. And yet virtually every piece of Mong Hsu rough is riddled with open fractures prior to treatment.
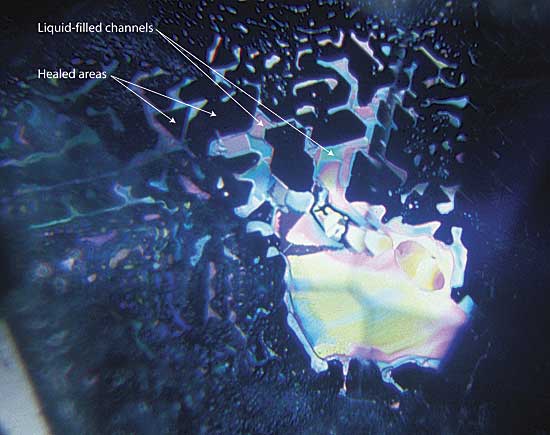
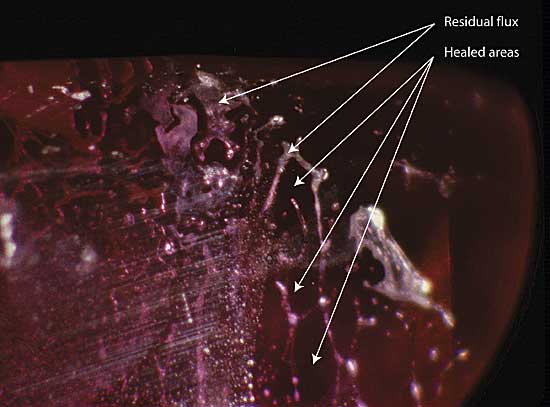 |
| Figure 8. Residual flux Residual flux in a flux-healed fracture within a heat-treated Mong Hsu ruby. The areas (in the plane of the fracture) between the flux-filled channels consist of healed ruby. Photo © R.W. Hughes |
The flux healing process
Flux healing involves heating corundums with borax or other fluxes. These fluxes actually dissolve the surfaces, including the internal surfaces of cracks. The corundum within this molten material then re-deposits on the fracture surfaces, filling and healing the fractures shut. Undigested material cools into pockets of flux glass. Essentially this amounts to a microscopic deposition of synthetic ruby to heal the cracks closed.
In the broadest sense, this is akin to the oiling of emerald—both treatments involve reduction of reflections from included cracks/fissures. Similar to placing an ice cube in water, a filled fracture is much less visible because the filler replaces air (RI = 1.00) with a substance that has an RI that more closely matches the gem itself (1.76–1.77). However, the flux healing of Mong Hsu rubies differs in three important respects:
- The Mong Hsu ruby treatment is not a fracture filling, but a permanent healing of the fractures and fissures, with any filling merely a remnant of the process. In many respects, it is a welding of fractures, similar to the joining of two pieces of metal with heat and a flux to lower their melting point.
- The Mong Hsu ruby treatment is permanent and irreversible. Unlike the oil in an oiled emerald, flux remnants will not drain out in the future, nor can they be removed. There is no way to have a stone revert back to the untreated state.
- The Mong Hsu ruby treatment actually improves a stone’s durability, since the fractures are permanently healed shut.
Dealing with it
With the explosion of Mong Hsu ruby onto the market, it became obvious that traditional lab nomenclature was not equipped to deal with this treatment. Thus in 1997, while directing the colored stone identification department of the Los Angeles office of European Gem Labs (EGL), I developed terminology to honestly describe this treatment. The idea was to provide the customer with an estimate of how this treatment had impacted the gem. A number of labs (AGTA, GIA, Gübelin, SSEF, GIT, GAAJ) have now adopted elements of this nomenclature and refined its application (see Lab Manual Harmonization Committee, 2004). The author’s suggested nomenclature is as follows and it can (and should) be applied to other treatments:
- Treatment type: Indications of heating + flux healing of fractures
- Extent: Minor/moderate/significant number of flux-healed fractures
- Stability: Stable/unstable under normal wearing conditions
- Prevalence: Never/rarely/commonly/usually/always found in the market
In the case of glass infilling, the size of filled cavities is important, with larger fillings having a greaterimpact on the appearance and weight of the stone.
The opposite is the case with flux healing. The more perfectly the treatment is
applied, the less residue that might be present in the healed fracture.
Summary
We have a superior treatment for Mong Hsu ruby, one that is actually more stable than ordinary oiling. So why worry?
First, purchasers of ruby are not accustomed to buying heavily fractured stones. Unlike emerald, clean rubies do exist in nature. Second, the process can also be accomplished with heat alone. If such stones are deemed acceptable without further comment, what happens when only heat is used?
In the end, the flux-healing treatment should be looked at for what it is, a radical reconfiguration of the clarity characteristics of a gemstone. If lumped together with simple heat treatment, it will completely redraw the map not just for ruby, but also potentially for the entire gemstone industry (think emerald here).
We must stop kidding ourselves. In the eyes of the consumer, the high-temperature heating and flux healing/impregnation of a ruby is not the same as simply cutting and polishing it. No amount of explaining will make it so. A gem that only requires polishing to reveal its beauty is far more rare than something that needs both polishing and ordinary heating. And that is more rare than the Mong Hsu ruby, which needs polishing, high-temperature heating and flux-fracture healing. The market should reflect these realities in its descriptions of goods and, most importantly, in it’s pricing. Gems and jewelry are luxuries. In the retail market, they compete against a number of different goods and services. If we don’t make clear distinctions between our different products, that retail customer may stop buying more than just Mong Hsu rubies.
• • • • • •
Postscript: Yehuda-type Pb-glass filled rubies
In March of 2004, the Gemmological Association of All Japan (GAAJ) encountered a treated ruby in which the fractures were filled with lead (Pb) glass. This is similar to the Yehuda fracture filling treatment of diamond. World gem labs have now seen several such stones. Like the glass-filled diamonds, Pb-glass fracture filled rubies show an iridescent “flash” effect on filled fractures when examined with oblique illumination under magnification.
Appendix: A Scientific View of Flux Healing |
||||
|
By John L. Emmett Many gemologists do not clearly distinguish between melting and dissolving. Melting is a definite property of a crystal which occurs at a definite temperature. For example, if you take a piece of ice at -50°C, and gradually add heat to the crystal, the temperature increases until it reaches O°C. Even if you continue to add heat, the crystal stays at 0°C and begins melting. As more heat is added the temperature remains at 0°C until all of the ice is melted to water, at which point the temperature will again increase with heat addition. For corundum this process occurs not at 0°C but at 2045°C. Below this temperature corundum does not melt. Table salt melts at 804°C. It works just likes the ice example above. Below 804°C there is no melting of salt. However salt can be rendered into a liquid form by dissolving it in water at temperatures far below its melting point. Salt dissolves in water at almost any temperature where water is a liquid, and slightly below. So now we have two points:
Thus melting and dissolution are two entirely different processes. Of solvents and solutes This is incorrect. With the types of ovens typically used for heat-treating gems, the crucibles and furnace muffles would break down before corundum’s 2045°C melting point was reached. In reality, no melting took place. Dissolution occurred. The stones were exposed to a material that acted as a flux (a high temperature solvent) that dissolved the corners off the stone. This could happen with some fluxes at temperatures well below 1000°C. Now I need to say more about solvents and solutes. At any given temperature, a given solvent will dissolve a certain maximum amount of solute. That is, there will be a fixed amount of solute per liter (e.g. grams/liter). Now we need some terminology. If a solution at a given temperature contains less solute that the maximum, it is called undersaturated. If it contains exactly the maximum amount, it is called saturated. If it contains more than the maximum amount, it is called supersaturated. For most solvent-solute combinations the solubility (grams/liter) increases with increasing temperature, or decreases with decreasing temperature. If a crystal is put in an undersaturated solution (the solute is the same as the crystal) it will slowly dissolve. If it is put in a saturated solution, it will grow at the same rate as it will dissolve. Thus the weight will not change. If it is put in a supersaturated solution, the crystal will slowly grow. How can a solution become supersaturated? One simple way is for the solution to become saturated at one temperature and then be cooled to a lower temperature. Since the concentration of solute for saturation is lower at the lower temperature, the cooled solution is now supersaturated. Supersaturation is not stable indefinitely; the excess will eventually solidify by crystallizing out of solution.
Into the void Why does this happen? The principle is that the shape will change to minimize the surface free energy. (Another example of minimizing the free energy of a system is that given a chance, water will always flow down hill.) What shape has the minimum free energy? Spheres are a lower free energy than very thin tubes. Spheres are also a lower free energy than a flat crack with a sharp point. In crystals, negative crystals with low Miller index faces are lower energy than spheres, etc. This topic is well covered in Roedder (1984) and references therein.
How does the material move to change the shape? In our example of a void in a synthetic crystal, there are two processes: surface diffusion of the atoms of the crystal, andevaporation and condensation of the crystal material. Flux healing Now let’s talk about the flux itself. Not all the corundum dissolved in it comes from the wall of the crack. The flux could have dissolved some corundum from the exterior of the stones or crucible before entering the crack, or corundum could have been deliberately added to the flux prior to treatment. Thus corundum can be transported into the crack from outside, as well as being dissolved from the walls. Chillin’ What is the solid flux like, crystal or glass? Depending on the cooling rate it could be either, or some of both. Slow cooling favors crystals, while more rapid cooling favors glasses. If the flux was originally a borate, the solid material in the crack is mostly an alumino-borate. |
Recommended reading
- Emmett, J.L. (1999) Fluxes and the heat treatment of ruby and sapphire. Gems & Gemology, Vol. 35, No. 3, pp. 90–92.
- Eppler, W.F. (1959) The origin of healing fissures in gemstones. Journal of Gemmology, Vol. 7, No. 2, April, pp. 40–66.
- Gemmological Association of All Japan (2004) Lead-glass impregnated ruby.
- Hänni, H.A. (1997–1998) Short notes on some gemstone treatments. Journal of the Gemmological Association of Hong Kong, Vol. 20, pp. 44–52.
- Hänni, H.A., Kiefert, L. et al. (1998) Ruby. In Standards & Applications, Basel, SSEF.
- Hänni, Henry A. (2001) Beobachtungen an hitzegehandeltem Rubin mit künstlicher Rissheilung (Observations on heat-treated ruby with artificially healed fissures). Zeitschrift der Deutschen Gemmologischen Gesellschaft, Vol. 50, No. 3, pp. 123–136.
- Hughes, R.W. (1984) Surface repaired rubies—a new gem treatment. Jewellery News Asia, p. 1.
- Hughes, R.W., and Galibert, O. (1998) Foreign affairs: Fracture healing/filling of Mong Hsu ruby. Australian Gemmologist, Vol. 20, No. 2, April–June, pp. 70–74.
- Lab Manual Harmonization Committee (2004) LMHC Information Sheet #1: Standardised Gemmological Report Wording (implementation beginning February, 2004)—Corundum with residue from the heating process present in healed fissures and/or cavities. 2 pp.
- Peretti, A. (1993) Foreign substances in Mong Hsu rubies. JewelSiam, Vol. 4, No. 5, p. 42.
- Robinson, N.L. (1995) Thais get burned by glass fillings. Colored Stone, Vol. 8, No. 4, July/August, p. 1, 6 pp.
- Roedder, E. (1962) Ancient fluids in crystals. Scientific American, Vol. 207, pp. 38–47.
- Roedder, E. (1984) Fluid Inclusions. Reviews in Mineralogy, Washington, DC, Mineralogical Society of America, Reviews in Mineralogy: Vol. 12, 646 pp.
Acknowledgements
The author wishes to thank William Larson, Josh Hall and John Emmett for fact-checking the manuscript.
About the authors
Richard Hughes is the author of the classic Ruby & Sapphire and over 100 articles on various aspects of gemology. His writings can be found on his personal web site, www.ruby-sapphire.com.
Dr. John Emmett is one of the world’s foremost authorities on the heat treatment, physics, chemistry and crystallography of corundum. He is a former associate director of Lawrence Livermore National Laboratory and a co-founder of Crystal Chemistry, which is involved with heat
treatment of gemstones.
Notes
This article came about because of discussions I had with The Guide’s Stuart Robertson. As I explained the concept of flux healing to Stuart one day, he remarked that, while familiar with the term, he never really understood the process and its impact on a stone. Thus I decided to revisit the issue, using illustrations that would make the subject crystal clear. The article was penned in July, 2004. Edited versions of the article appeared in The Guide and IDEX, Sept. 2004.
_______________
[1] It was later published in the Australian Gemmologist (Hughes & Galibert, 1998), and can be seen at this URL: http://www.ruby-sapphire.com/foreign-affairs.htm.
[2] Note that this acid is extremely dangerous and should only be used under controlled laboratory conditions.