 |
Grafting was an academic model of natural pearl nucleation—the patents that made pearl culture also made a point toward finding the natural origin of pearls. Over the century since, some of the ideas then on the table for the matter of pearls, turned out to be important, changing much of biology [1]. The origin of natural pearls is worth revisiting, taking the discussion from where it was left.
The invention of grafting came around 1907 [2] out of a race for the origin of pearls. At the time, the biological event of natural pearl nucleation was quite an academic matter: much was written on the formation of shell, most translated into possibilities for natural pearl nucleation rather than convenient pearl culture, until one thing worked. Grafting proved that nacre maker cells are necessary to induce nacre production elsewhere among an oyster’s anatomy despite the different context upstream. However, it remained unclear how these turned up at natural pearl nucleation sites, and whether, albeit insufficient for nacre production, simpler mineralization was a necessary primer toward cellular specialization for nacre making.
The fairly colorful exchange between Jameson [3] and Herdman [4] sums up much of the thinking that turned the search for increased pearl production from dependable field biology to then tentative notions of cell specialization [5].
I cannot quite be sure that mine are the first images of natural pearl nuclei since electron microscopy came about… It seems improbable that they had vanished from the ever-increasing mass of academic work on nacre and shell materials. These are some of my samples:
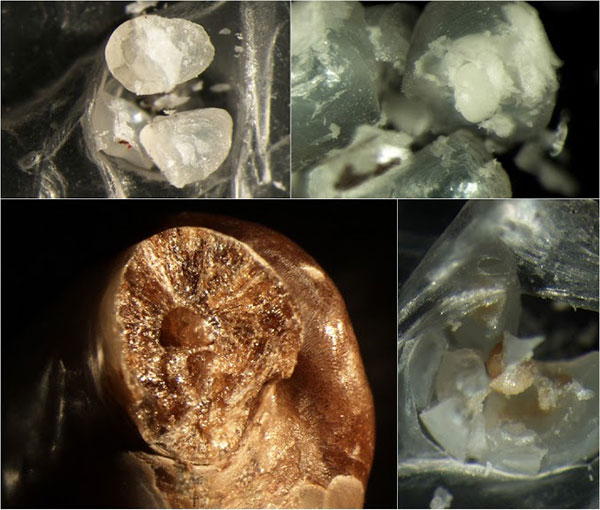 |
| Figure I Top right: Pinctada radiata – nacre looks transparent in section, the dull white mineral mass in the middle is what started this pearl, there are multiple centers of radial fibrous aragonite among rougher, layered material; see Figure III radiata fibrous, the bottom strip, for a closeup. Top left: Pinctada radiata – also of colorless, very translucent nacre. Early mineralization forms a compact granule that has detached; it is made of a ‘flaky’ mineralization, also found in letter growth remaining on the ‘socket’ from where the nucleus/granules have detached. There is an interesting transition between this material and nacre—details in Figure III radiata detached. Bottom left: Multiple nuclei in a baroque prismatic Penn pearl. Prismatic shell material forms the exterior layer over nacre in all classical pearl making species—the Pterias, Pinctadas… Penn shells are also nacro-prismatic. In all such species, pearls may form of either prismatic material, or, somewhat unusually, both. This baroque Penn pearl is partially covered with nacre [not shown]. A couple of Pinctada radiata pearls with partial nacre covers over prismatic material are shown in Figure IV. Bottom right: Another cleanly detached nucleus from a P. radiata pearl—unlike the one above, this is formed of relatively large mineral grains interspersed with organic material, hence the color. Samples held at the University of Granada. Source: Mattar Jewelers [P. radiata], K.C. Bell Natural Pearls [Penn]. |
The mineral grains that come cleanly out of some nacreous pearls do not appear much in literature for good reason: these separate if you crack the pearls under very slight, even pressure—not exactly standard procedure. More commonly, natural pearls are cut and the sections polished—in such preparations, my granules would appear as central regions of consistent mineralization bounded by some structural discontinuity with letter growth... There are reasons to do things either way.
Although various extraneous objects could be found inside pearls, these samples were the exception to the rule of finding nothing at all. By 1912 Jameson reconsidered this majority of ‘pearls without nuclei’ [6] to be the more informative; his survey of natural pearl nuclei and sacs—seriously rare observations in literature!—are the best I have in print. Sure enough, samples are even better, and I am finding in them much the same as Jameson shows in his plates.
Up to the time of Jameson and Herdman, many natural pearl samples were decalcified to look for foreign objects expected to have caused pearl growth; they also favor this method [7] that lost them little: not much could be said about the mineral side of pearls until X-Ray crystallography could handle such samples [8]. If no organic structure survived at the center of natural pearls, they deserved the label of pearls without nuclei that Jameson gave them; the ones in my pictures would have fit the bill.
It is somewhat surprising that the pearls without extraneous nuclei were not nearly as much discussed as the few others with something more obvious to show in their middle. The earliest report I have of pearls found to be empty comes from 1717 [9]—and it was still being cited in the 1900s. Perhaps it was simply common knowledge that the common pearls did not suggest any convenient mechanism of nucleation, and what everybody knows that everybody knows is somewhat difficult to read between the lines.
Field work pursued an honest hope:
It was concluded ‘such methods of artificially promoting natural infection [with—wishfully—parasites inducing pearls] would be incomparably superior to any method of pearl production by operation on the individual oyster…’. [Jameson, 1902, 163] [10]
Four years later, Herdman’s book buttresses the same—‘remarkable suggestion […] that it might be possible to increase the quantity of pearls by infecting the oysters in other beds with the larvae of pearl-producing parasites…’ [Herdman, 1906 Part V, 7] [4]
Oddly, there is no shortage of external factors that do induce pearls—all as unreliably as they do today [11]. Infesting organisms, be it the much sung parasites or algae [12], slight shell deformations, adductor displacement… are associated with natural pearls some times but not nearly all the time; same can be said about anomalous mineralization other than pearls – it is sometimes associated with a successful healing process, but not necessarily [13]. Happenstance as natural pearl nucleation is, apparently all but none of the pearls nucleated grow to fine size: what makes the difference is yet another question [14]—see Figure II. Such details must have become obvious in the field, if not necessarily glaringly stated in the field reports. I am intrigued whether any substantial trials were attempted in Ceylon or elsewhere…
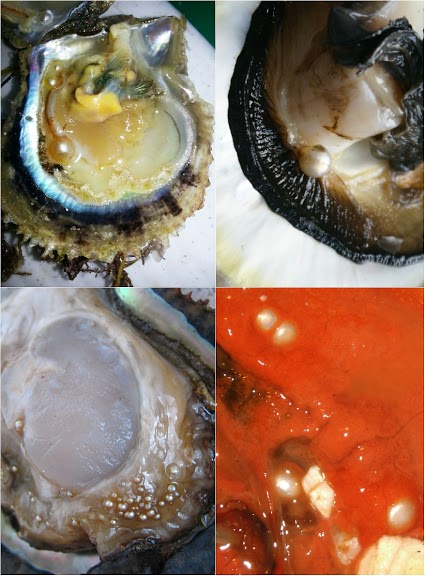 |
| Figure II Small pearls—sometimes spectacularly many to the shell; large pearls rarely more than one: they might follow entirely different paths of growth… Although a few larger Pinctada radiata pearls are available for destruction, I have only opened one to inspect its nucleus: the pearl was the only one I have cut and polished as it is usually done, cleaning away some interesting modified nacre close to what must have been its nucleation site. Some procedure to peel off letter growth would be welcome for the remaining samples. Top right: Fine pearls in a Bahraini Pinctada radiata shell [source of image: GPTLB (The Gem and Pearl Testing Laboratory, Bahrain); personal communication]. Top left: A fine pearl grown next to the adductor [source of image: GIA, Bangkok; personal communication]. Possibly similar to the ‘nacreous muscle-pearls’ described in Jameson (1912 [3], page 324). These samples are not held at the University of Granada. Bottom left: Golden pearls pepper the mantle of a Mexican Pteria sterna [source of image: Douglas McLauren, Perlas del Mar de Cortez]. Bottom right: Minute pearls in a Californian blue mussel [source of image: David Leblanc]. These latter two sets of pearls are available for study. One of the Pteria sterna goldens appears in my upcoming paper on natural pearl nucleation. |
By 1906, Jameson had changed his mind—if nothing in particular triggered pearl formation, external factors were the wrong thing to study:
the essential element in pearl-formation is the pearl sack and not the nucleus […] it is by a study of the causes that led to the development of the former that the problem of the origin of pearls is to be solved.
However, inducing some cell function was a technical challenge of an entirely different order than schlepping oyster parasites from one pearling field onto another. That cells could acquire some function unexpected in the surrounding tissue was more of a philosophical than technical matter. Cells with the right kind could be moved—grafting did this—and were thought if not seen to do so in nature.
Herdman [4] cites from Jameson’s letters:
I had never any doubt that it [the lining of a nacre-producing pearls sack] is a true epidermis, but I never got so far as to determine actually by observation whether it arose, as I think you have suggested, by the Trematode carrying in with it a fragment or pocket of epidermis; or, as I suspected, by means of epidermal or sub-epidermal replacement cells (Ersatz-zellen). [15]
The question of the sack’s origin was not new. [16] On the second page of his 1912 book, Jameson announces no progress:
the essential element in pearl-formation is the pearl sack […]. Owing to difficulties and delays which may occur, [I publish] without attempting to deal with the origin of the pearl-sack. [17]
Jameson’s experiments with epithelial specialization [18] were done on shell repairs [see Figure IV and its discussion]—it is unclear how he might have hoped to advance [19]. Herdman did not claim his counterargument to be held for any stronger reason but acceptability:
It is very probable that the parasite in burrowing into the mantle carries in with it one or more epidermal cells which proliferate to form the [pearl] sac.[...] Even in the absence of direct evidence of this, it will be admitted that it does not involve such a violent assumption as that the connective tissue in the centre of the mantle [in section] can produce an epithelial sac, the cells of which are indistinguishable both in structure and in function from the epidermis outside.
Simply by keeping closer to well trodden references and, implicitly, to contemporary technical possibilities, Herdman’s view rather fit the grafting scenario of cell migration by an exogenous agency. Jameson’s assumption is no longer surprising, if a good call for ectopic shell production might be a task precisely as intense as the same exploration of bone… [20, 21]
Grafting left an interesting assumption somehow beyond discussion: that cell specialization toward shell production is equivalent to its ability to contribute to the recognizable organic structure included in them—the same material that served to identify the remains of shell materials in the decalcified samples of yore, the same seen to form somewhat ahead of mineralization on the shell… Not a lot is known of how mineral supply and this specialized secretion play along in cells—and perhaps this is the academic point that natural pearl nuclei make today: that things may start with the cellular handling of mineral supply. The idea was almost there in Jameson’s finding great variation in pearl growth until the new material acquires shell-like structure. Much to be said…
The last time anyone looked at natural pearl nuclei, electron microscopy was not around. I suspect these are the first such shots of natural pearl nuclei:
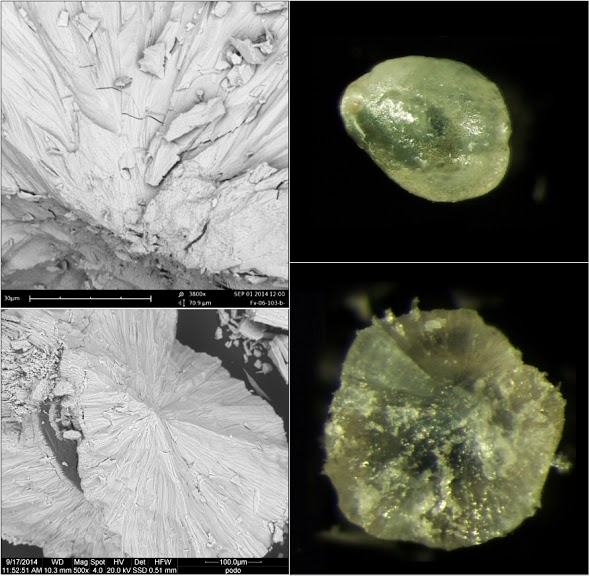
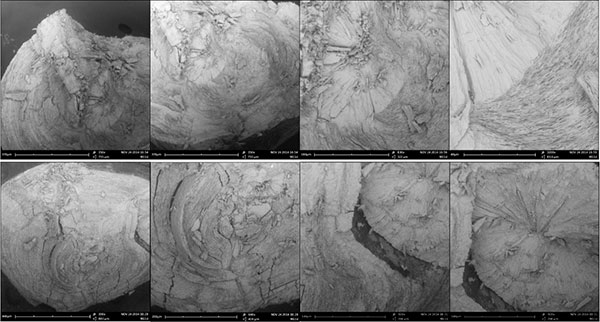
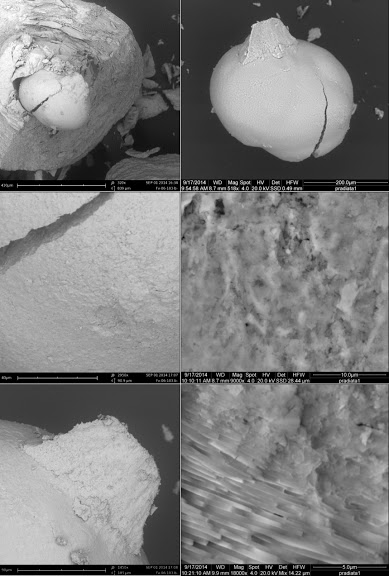 |
| Figure III (A) A Pododesmus fibrous aragonite, ‘muscle pearl’. This is one of several ~1-mm pearls formed right at the edge of the adductor attachment of a Pododesmus macrocisma upper shell. The position is ambiguous—the pearls could have been associated with shell production, or with the mineralization produced around the attachment of muscle fibers onto the shell—the myostracum [‘hypostracum’ in Jameson [3]]. In other species, it might be difficult to tell which is what since the rougher myostracum material may resemble some generic anomalous mineralization… However, the Pododesmus shell is entirely made of calcite, except for the myostracum which is fibrous aragonite, as are these pearls. Reportedly, letter growth of such pearls turns to shell material. The same is reported in the older literature about classical pearls: some may come with myostracum centers… perhaps not unlike the one in Figure II, top right. Top right: Sheaves of fine aragonite fibers radiating from the center of the pearl. Bottom right: Fibers extend from the center to the surface of the pearl, occasionally interrupted by lenticular deposits of a deep green organic material that stains the pearl. Top right: The pearl as it were. Bottom right: A shard of it… (B) Shards of two Pinctada radiata pearls with fibrous aragonite nuclei. The fibrous aragonite is not organized precisely as in Pododesmus—fibers are not bundled in sheaves… but such details may never be apparent from non-destructive observations of pearls. (C) A detached P. radiata nucleus—the same as in Figure I, top right. Here: Top left: Detail of Figure I, top right in the scanning electron microscope. Top right: The nucleus, detached. Mid right: The edge of the crack in the nucleus showing consistent grainy mineral fabric over the rim; 9KX close-up in the mid left image. Bottom left: A shard of letter mineralization remains attached to the nucleus; it is made of the same material, and still the same is found around the ‘socket’ from where the nucleus has detached. Bottom right: Transition between nucleus material and nacre toward the outer rim of the ‘socket’, 18KX. Samples held at the University of Granada. Source: David Leblanc [Pododesmus], Mattar Pearls [P. radiata]. |
Most of the images above are lazy electron microscopy—the shots are just at the lowest magnification possible—~400X, giving a clean panoramic view that great photomicrography might as well best [yes, this is an invitation…]. Details shot around ~10–20KX [ESEM] are clearer in context; please see a couple of close-ups of the transition between nacre and the disordered ‘nucleation’ mineralization in the Pinctada radiata nucleus shown detaching in Figure I—what Jameson assumed to be shell repair material, but I am not as ready to. [22]
In some form, many of the other broad concepts of shell mineralization that were around in the background of the literature on natural pearl nucleation, are still around. [20] The correspondence is somewhat philosophical—much more detail is accessible, of course. Also, more can be said about the structure of natural pearl nuclei, not just because tools are better, but because shell materials are better known. Easily, the earliest ‘primer’ mineral fabric formed, initiating run-of-the-mill shell layers, has its correspondence in natural pearl nuclei. Interestingly, natural pearls that Jameson knew better than are known today do, in fact, call for the ‘violent assumption’ of cell specialization plasticity twice: first toward mineralization at nucleation, then to switch between different shell materials—these days it is understood that different shell materials are made by cells of slightly different looks, but very different specialization. [21]
 |
| Figure IV Pinctada radiata pearls of prismatic shell material [the outer layer of the shell] and nacre. Bottom left: Some of the batch of minute Pinctada radiata pearls I am using; the P. radiata pearls above were among these… Please notice the white and golden nacre, and a dark, fully prismatic pearl. The range of nacre’s qualities is apparent even in these small pearls; mineral structure varies subtly, often surprisingly. Breaking many pearls it becomes apparent that the much sung mechanical properties of nacre vary sample to sample as well. Much to be said! Top right and left: A drop pearl of prismatic material with partial nacre cover. This is an unusual, single nacre front. The gradation of brown reflects the extent of organic inclusions. Bottom right: Another drop, with much more extensive nacre cover [nearly transparent]. Jameson describes both prismatic aragonite and prismatic calcite pearls with full, fine nacre covers… It is difficult to say whether the prismatic or fibrous aragonite in such pearls is analogue to a shell material, possibly the myostracum, or may be a somewhat unexpectedly well structured anomalous mineralization produced along the pathway of secretory specialization toward nacre. However, the prismatic calcite of these pearls is intuitively analogous with the outer shell layer. Shell-making cells are thought to be able to switch between secretory regimes [a subject of current research [21]]… Samples held at the University of Granada. Source: Mattar Pearls. Optical microscope images taken at the University of Bourgogne, Dijon, with the help of Frédéric Marin, Jérôme Thomas and Christophe Durlet (COST STSM, December 2012). |
Now as then, taking up assumptions on the formation of shell materials to bear on natural pearl nucleation, would test them in a new context where dependencies among the various processes coordinated for shell production, may be unraveled and exposed. Sure enough, today it would be possible—and great fun—to update the detailed plates of H. L. Jameson and others, with beautiful microscopy showing what is possible in natural pearls, nudging at the boundaries of what shell fabrication can deliver… It might be tempting to look again at the state of natural pearl fisheries and revisit the original task for which the work of Herman and Jameson was contracted in the first place—to find and to favor the natural conditions yielding fine pearls, ‘incomparably superior to any method of pearl production by operation on the individual oysters’. Truth is worth more than pearls—as the saying goes, so perhaps this truth about pearls is not worth the hassle.
Still… it is good to know that natural pearls happen mostly otherwise than by a natural counterpart of grafting, that they embody an archaic capacity of regeneration, that their depth is no illusion… [23]
Notes
1. I am thinking of cellular specialization plasticity and signalling—neither idea was quite accepted at the time of the natural pearl nucleation debate. Henry Lister Jameson [3] proposed that ‘reserve cells’ suitable for regeneration [‘Ersatz-zellen’] could specialize ectopically for nacre production forming pearl sacks, but his was an unusual and technically perfectly inaccessible approach.
Herdman deems the idea ‘a violent assumption’. [4] Cell specialization plasticity will come back in the discussion of natural pearls in Haynes (1924). These days, the process is best understood for bone mineralization.
Sources:
Haynes, T. H., “Notes on the growth of molluscan pearls and shell and on Pholadidea parva causing blisters in Haliotis”, Proceedings of the Malacological Society, London, Vol. XVI (1924), No. 3: 112–121.
For a connection between current research on shell and bone mineralization, see:
Marin, Frédéric, et al., “Mucins and molluscan calcification: Molecular characterization of mucoperlin, a novel mucin-like protein from the nacreous shell layer of the fan mussel Pinna nobilis (Bivalvia, Pteriomorphia)”, Journal of Biological Chemistry 275.27 (2000): 20667–20675.
2. The year of Tatsuhei Mise’s patent.
3. Jameson, Henry Lyster. “Studies on Pearl-Oysters and Pearls.—I. The Structure of the Shell and Pearls of the Ceylon Pearl-Oyster (Margaritifera vulgaris Schumacher): with an Examination of the Cestode Theory of Pearl-Production.” Proceedings of the Zoological Society of London, 1912. (archived here)
4. Herdman, W. A. “Report to the Government of Ceylon on the pearl oyster fisheries of the Gulf of Manaar”, The Royal Society, 1906. (archived here)
5. Signalling and its relation to cell specialization would have been critical concepts, but neither was around at the time. The first signalling secretion had been reported in 1902 by Bayliss and Starling—the concept was still disputed and quite far from any connection to cell specialization. That cells may change function, or new cells could acquire functions unlike their near neighbours, was not mainstream: Haynes (1924 [1]) notes some of the first experiments challenging these views, without noticing the connection with Jameson’s proposal [3] which he does cite…
Source:
Bayliss, William Maddock, and Ernest Henry Starling. “The mechanism of pancreatic secretion”. The Journal of Physiology, 28.5 (1902): 325–353.
6. Please see the section on ‘Pearls without nuclei’ in Jameson (1912 [3], 266–267) and his definition of ‘pseudo-nuclei’ corresponding to these granules in my Figure I (page 321), and ‘Pearls and parasites’ in Haynes (1906 [1]).
7. Methods in Jameson (1912 [3]) and Herdman (1906 [4]), inter alia.
8. For early applications of X-Ray crystallography to natural pearls and shell, see C. V. Raman references below. S. Rama Swamy, “X-Ray analysis of the structure of iridescent shells”, Proc. Ind. Acad. Sci., Section A, Vol. 1 (1935): 871–879, cites sources going back to the early '20s. Interestingly, this early literature phrases the still interesting problem of reconciling the crystallographic coherence and segregation patterns of shell materials. For pearl gemmology see:
J. H. Shaxby, Comptes Rendus Vol. 179 (1924), 1602–1603. (archived here)
Dauvillier, Comptes Rendus, Vol. 179 (1924), 818. (archived here)
J. Gaiborg and F. Ryziger Comptes Rendus Vol. 183 (1926), 960–962. (archived here)
The last notes interestingly high crystallographic coherence of some natural pearls appearing as ‘single crystals’. It would be interesting to know how good this approximation can get— for a current view of a pearls crystallographic coherence, see Erika Griesshaber, Wolfgang Schmahl, “EBSD on the Nacre Structure of a Pearl (Hyriopsis cumingii) with 100 nm Resolution”, Oxford Instruments publication, 2012. (archived here)
9. Réaumur, René Antoine Ferchault de, “Sur la formation des perles”, Mémoires de l’Académie des Sciences, 1717: 177–194. (archived here)
10. Jameson, Henry Lyster, “On the origin of pearls”, Proceedings of the Zoological Society of London, 1902: 140–166. (archived here)
11. Many recent field reports of natural pearl incidence are done on blue mussel populations.
12. Rodríguez, F. et al., “Phylogenetic and morphological characterisation of the green algae infesting blue mussel Mytilus edulis in the North and South Atlantic oceans”, Dis. Aquat. Organ., Vol. 81 (2008): 231–240.
Galimany, E. et al., “Pathology and immune response of the blue mussel (Mytilus edulis L.) after an exposure to the harmful dinoflagellate Prorocentrum minimum”, Harmful Algae, Vol. 7 (2008): 630–638.
Zuykov, M. et al., “First record of the green microalgae Coccomyxa sp. in blue mussel Mytilus edulis (L.) from the Lower St. Lawrence Estuary (Québec, Canada)”, Journal of Invertebrate Pathology, Vol. 120 (2014): 23–32.
13. Trinkler, Nolwenn, et al. “Mineral phase in shell repair of Manila clam Venerupis philippinarum affected by brown ring disease”. Diseases of Aquatic Organisms, 93.2 (2011): 149. (archived here)
14. Jameson (1912 [3]), page 332 has an interesting idea: ‘Probably such a pearl would…’. I do not know whether the small pearls often found by the dozen in one shell—to put it mildly; my most pearl-ed sample had eighty—have much to do with the fine pearls that are not often crowded. Open for consideration…
15. Jameson does not mention a source for his association of ‘replacement cells’—a historical precursor of the concept of stem cells—and natural pearl nucleation; his model of natural pearl nucleation is inspired by shell repairs [5], but no further discussion of ‘Ersatz-zellen’ precursor cells appears. I have not reviewed Jameson’s German sources.
16. Hessling 1858, Diguet 1899, cited in Herdman (1906 [4]), page 8.
17. Jameson (1912 [3]), page 261.
18. Jameson (1912 [3]), page 275: ‘My chief grounds for doubting the Cestode theory…’
19. Having established the presence of cells retaining secretory plasticity at natural pearl nucleation sites, an ‘agency’—in Jameson’s terms, or an appropriate trigger for their specialization would be required; at page 270, Jameson (1912 [3]) recounts that his 1902 paper concludes that a ‘specific stimulation’ is key. Between 1902 and 1912 it had become clear that the specific factor, which induced the right kind of ‘parenchyma’ epithelial lining around pearls, was not external. This is still a tall order: for an overview of the corresponding challenge identifying the controls of bone morphogenesis, see Rodan, Gideon A. and Shun-ichi Harada, “The missing bone”, Cell 89.5 (1997): 677–680.
20. For example, the idea of whether upstream mineral supply—generated away from the shell—might be sufficient to form at least some shell materials, also as natural pearls… or whether cell migration plays a part, be they mobile cells or modified epithelial cells, ironically, observed in pearl grafts.
For some cell migration involved in shell mineralization, see:
Mount, A.S. et al., “Hemocyte-mediate shell mineralization in the Eastern oyster”, Science 304 (2004): 297.
Johnstone, M.B., Ellis, S., Mount, A.S., “Visualization of Shell Matrix Proteins in Hemocytes and Tissues of the Eastern Oyster, Crassostrea virginica”, Journal of Experimental Zoology, 310B (2008): 227–229.
Mount et al., “Deposition of nanocrystalline calcite on surfaces by a tissue and cellular biomineralization”, United States Patent Application Publication US 2013/0251968 A1,2013. (archived here)
And the same mediating the spread of modified epithelial cells of a graft, described in:
Masahiko Awaji, Akira Machii, “Fundamental studies on in vivo and in vitro pearl formation—Contribution of outer epithelial cells of pearl oyster mantle and pearl sacs”, Aqua-BioScience Monographs, Vol. 4 (2011), No.1: 1–39. (archived here)
21. Hence difficult to tell apart by their looks, as could be done back in the day, but well distinguished by tracing the type of organic shell components they produce. For Jameson’s discussion of differentiated shell production see: Jameson (1912 [3]), (12), page 310; for the update see: Marie B., Marin F., “Different secretory repertoires control the biomineralization process of prism and nacre deposition”, PNAS, Vol. 109 (2012), No. 51: 20986–20991. Jameson and others also noted that in repairs shell making cells must change their operative regime between the two types of material—prismatic and nacre—deposited, but the change could not be observed; for an update see: Shun Aoki, “Comparative histological observations on the pearl-sac tissues forming nacreous, prismatic and periostracal pearls”, Bulletin of the Japanese Society of Scientific Fisheries, Vol. 32 (1966), No. 1: 1–10. (archived here)
22. For a connection between shell repairs and grafting, see Masahiko Awaji, Akira Machii, “Fundamental studies on in vivo and in vitro pearl formation—Contribution of outer epithelial cells of pearl oyster mantle and pearl sacs”, Aqua-BioScience Monographs, Vol. 4 (2011), No. 1 (archived here). There is much written about shell repairs, but without reference to natural pearls. I will discuss this comparison between shell repairs and natural pearl nucleation in an upcoming paper. The idea has always been attractive, since the beginning of my look at pearls, when anomalous versions of shell materials both in shell repairs and in natural pearl simply seemed just as much of a mess…
23. I am referring to a particular illusion—the imaginary depth that light gives to nacre. Please, backlight a fine pearl as Raman did. The references are somewhat rarely visited these days:
Raman, C. V., and D. Krishnamurti, “The structure and optical behaviour of pearls”, Proceedings of the Indian Academy of Sciences, Section A, Vol. 39 (1954), No. 5: 215–222.
Raman, C. V., and D. Krishnamurti. “On the chromatic diffusion halo and other optical effects exhibited by pearls”, Proceedings of the Indian Academy of Sciences, Section A, Vol. 39 (1954), No. 6: 265–271.
Raman, C. V., and D. Krishnamurti, “Optics of the pearl”, Current Science 23.6 (1954): 173–176.
Best read with the authors’ take on shell nacre:
Raman, C. V. and D. Krishnamurti, “The structure and optical behavior of iridescent shells”, Proc. Indian Acad. Sci., Section A, Vol. 39 (1954), No. 1: 1–13.
Raman, C. V., “On iridescent shells. Part I Introductory”, Proc. Ind. Acad. Sci., Section A, Vol. 1 (1935) No. 9: 567–573.
Raman, C. V., “On iridescent shells. Part II Colours of laminar diffraction”, Proc. Ind. Acad. Sci., Section A, Vol. 1 (1935), No. 9: 574–589.
Raman, C. V., “On iridescent shells. Part III Body-colours and diffusion-haloes”, Proc. Ind. Acad. Sci., Section A, Vol. 1 (1935), No. 12: 859–870.