|
Note: Since December 2001, treated sapphires from Thailand have created a great deal of controversy (see ‘Questions About Treated Sapphires from Thailand’ and ‘Understanding the New Treated Pink-Orange Sapphires’ for full details). The evidence is now overwhelming that such stones owe their color to a form of outside-in diffusion of coloring agents. This process is basically the same as that used with lattice diffusion treated blue sapphires in the past, but with different chemicals. Both the AGTA and GIA labs are now labeling this treatment process “lattice diffusion” and that information will be clearly listed on their identification reports.
October 2001, Australian gemologist Terry Coldham informed the author of a new treatment for orange sapphire. His initial report was that a burner in Chanthaburi, Thailand had developed a new method to treat off-color Songea (Tanzania) stones to a fine orange to red-orange color. Shortly thereafter, several other sources confirmed the news and on Nov. 16 2001 we sent out an e-mail alert. The stones were to be marketed under new names, such as Sunset sapphire, etc.
AGTA Lab Director Ken Scarratt visited Bangkok in December 2001 and obtained samples. Pala International’s Bill Larson also purchased samples of these stones last December in Bangkok. When Scarratt examined his stones back in New York, he found that all had been exposed to high-temperature heat treatment. But many displayed features suggesting there might be more to this than a simple bake job. Unusual orange color rims surrounding pink cores.
On December 28, 2001, Scarratt asked the author if he knew anything about the stones, mentioning the orange color rims. We quickly examined the Pala stones just purchased in Bangkok and found identical color rims on most pieces. Shortly thereafter, the AGTA issued a Lab Alert and we sent a number of stones to the GIA for analysis. The following is based upon the AGTA Report of Jan. 8, 2002 and GIA reports of Jan. 28, 2002 and Feb. 16, 2002, along with discussions we have had with American experts, such as John Emmett, [1] and other dealers and gemologists around the world. (Note: new online reports by the GIA and AGTA were issued on April 19, 2002, with a further update on May 3, 2002.)
Color ranges and types
The finished color range of these goods runs the gamut from yellow through golden yellow, to orange (including the range that encompasses padparadscha) and even into borderline ruby colors, some of which resemble red spinels. What initially began as a treatment for Songea sapphires quickly spread to Madagascar pink sapphires, off-color Thai/Cambodian rubies and even green sapphires from Australia and elsewhere.
From what we can gather so far (and the situation is changing rapidly), pink Madagascar stones are treated to orange (including padparadscha colors), green sapphires from Songea, Thailand and Australia are being treated to golden colors, and off-color Songea orange and reddish sapphires and purplish Thai/Cambodian rubies treated to better, redder colors. The following report applies only to stones that show orange color rims.
Lattice-diffusion treated? Likely.
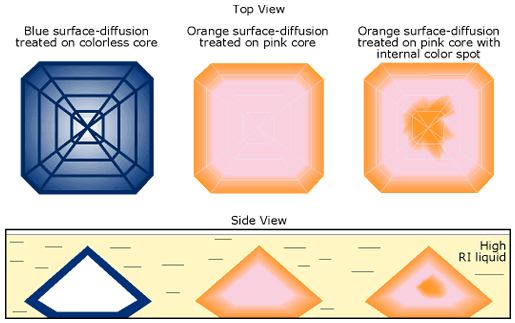
An unusual characteristic in many stones is a surface-based orange color layer surrounding a pink core (see photo below). Superficially, this resembles lattice (formerly termed ‘surface’) diffusion (see box below), but unlike previous lattice-diffusion treated gems, the facet junctions and girdle show no highlighting. Instead, what is seen is a layer of yellow-orange that follows the shape of the cut stone exactly. This suggests that at least the final portion of the treatment is applied to the cut stone, rather than the rough. It also suggests something being added from outside, because if it was simply a heat treatment acting upon elements already within the stone, the internal color pattern would not follow the shape of the cut stone exactly. There is no mine that produces rough orange sapphires in a perfect trillion shape (see below).
 |
| No, this gem does not come from the “trillion” mine, but is a Madagascar pink sapphire with an orange color rim created by lattice diffusion. The gem is immersed in di-iodomethane. Note that recutting such stones will produce a loss of the orange color. Photo: R.W. Hughes |
In addition to the now-common orange rinds on orange sapphires, sources have reported similar orange rims on rubies. Indeed, one told the author that burners in Thailand are now actively seeking off-color Thai/Cambodian rubies for color improvement via the added orange color rind. Similarly, those burners are said to be seeking old stocks of green sapphires for treatment to golden colors.
Recutting? Just say “no.”
We attempted to recut one emerald-cut orange stone, but stopped after just 0.12-ct. of weight loss when serious color loss was noted. Another source reported a similar loss of color during recutting. In other stones, the color appears to go all the way through. Dealers have reported recutting these color rind-free stones with little or no loss of color.
Golly, Molly, what are these things?
To answer that question, a meeting was held in Tucson on Feb. 5, 2002. In attendance were gemologists and dealers from around the world, including representatives from Thailand. Theories discussed included the following:
Zapped?
Initial reports suggested stones were possibly undergoing irradiation and that the color was unstable, fading with prolonged light exposure. However, the GIA’s Shane McClure pointed out that such irradiation would not color an entire stone’s surface equally, which is what appears with many of these new stones. Reports on fade tests have also resulted in no loss of color. Thus we can safely scratch irradiation as a possibility.
Geritol-rich gems?
Reports from two Thai labs pointed to an alteration of the valence state of iron from Fe2+ to Fe3+ and overall Fe content as the possible cause of the orange rims.
Discussions here in America suggest this is probably not the case. According to John Emmett, former Associate Director for Lasers at Lawrence Livermore National Laboratory, and one of the world’s top experts on the chemistry and physics of corundum, for iron to produce a yellow color in corundum, iron substitutions on the order of 2–3% are required. To the best of our knowledge, this is not being found in the pinkish orange stones with color rims.
Burned at the steak?
Yet another theory is that the stones are cooked “like a steak.” Gems with shallow color rims are equivalent to “medium rare” cooking while those with darker colors where the color goes all the way through are “well done.”
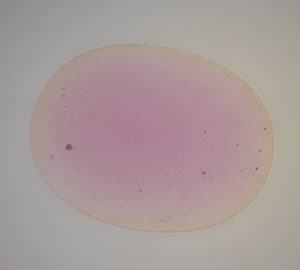 |
| Orange rim surrounding a pink core in a lattice-diffusion treated orange sapphire from Madagascar. The color rim is visible when the gem is immersed in di-iodomethane and is evidence of a treatment applied after cutting. Note that recutting such stones will produce a loss of the orange color. Photo: R.W. Hughes |
Again, John Emmett dismissed it, stating that corundum is essentially “isothermal,” meaning that it conducts heat so well that there is no significant temperature difference between the skin and center of a gem. Of the major gems, only diamond and silicon carbide have better thermal conductivity than corundum.
Synthetic overgrowth?
Perhaps the most bizarre theory on these color rims was that of Themelis.com, which in a briefly aired report suggested that they represented synthetic overgrowths of orange sapphire on pink sapphire cores. No evidence of this has been found. (Note: While we initially found no evidence of this, the latest AGTA report of April 19, 2002 suggests that dissolution of the gem surfaces by fluxes and redeposition of synthetic corundum may play a part in this treatment)
Showdown at the Tucson corral
With x-ray, iron, steak and fake discredited, it was left to John Emmett to explain. At the Tucson meeting, he described the most likely cause of the orange color rims as outside-in “lattice-diffusion” of a trapped-hole color center-producing ion. Such an element could be any small, light, allovalent ion from the upper left corner of the periodic table. Likely candidates are magnesium (Mg2+), beryllium (Be2+), calcium (Ca2+), lithium (Li1+), sodium (Na1+) or potassium (K1+). Even things like copper (Cu2+) and silver (Ag1+) could be involved.
According to John Emmett, at high temperatures the diffusion process draws elements present on the surface of the stone into the stone. At the same time, when this process is conducted in an oxidizing atmosphere, point defects called “holes” (which are the absence of an electron) are also created on the surface, and they diffuse much more rapidly throughout the stone. If some of these holes are trapped by the beryllium , magnesium, etc. which has diffused into the stone, they create what is called a “trapped hole color center.” In corundum, the trapped-hole color centers create a strong yellow coloration. This yellow coloration in a stone with a pink body color creates an orange coloration. However, not all stones will react the same way during this treatment. If titanium or other tetravalent impurities are present, they can bind with the magnesium or beryllium in such a way as to prevent formation of the trapped-hole color centers. Thus the relative amounts of the diffused-in element, and all the other impurities naturally in the stone, will determine the final color. This explains why individual stones react differently to the treatment.
Further evidence of a trapped-hole color center is the nature of the color itself. According to Emmett and Douthit (1993):
The strong orangey yellow coloration produced by this [Mg2+] point defect is very different from the pale pure yellow of iron-produced coloration.
The quantities required to develop such color centers are infinitesimal, as little as 20–30 parts per million. But this creates a further problem. Such levels are virtually undetectable, even for well-equipped labs such as the AGTA or GIA. In the end, the actual colorant may be undetectable with current technology.
A second problem is that, again according to our current understanding, so little of the allovalent ion may be required for this treatment that burners may not be aware that they are lattice-diffusing these stones.
 |
| This melted crystal with trapped gas bubbles in an orange sapphire believed to be from Songea, Tanzania, is strong evidence of high-temperature heat treatment. Photo: R.W. Hughes |
Science to the rescue
At the suggestion of John Emmett and Intel’s Gene Meieran, just before the Tucson 2002 Gem Fair the GIA subjected three different cross-sectioned samples to Laser Ablation Inductively Coupled Mass Spectrometry (LA-ICP-MS) and Secondary Ion Mass Spectrometry (SIMS) analyses. The GIA’s Shane McClure presented the results of the tests at the Tucson meeting.
While LA-ICP-MS turned up nothing unusual, SIMS analysis revealed unusually high amounts of beryllium (Be) in the orange color layer. Since Be is not normally found in corundum, and since the elevated Be levels of the skin were not found in the pink cores of the tested samples, the evidence is quite strong that, at least in some samples, the skin color appears to be due to lattice diffusion of light allovalent ions to create a yellow-producing trapped hole color center. The GIA’s findings can be viewed at this link.
Better living through chemistry?
While the jury is still out on stones where the color goes all the way through (generally golden and rich red-orange in color), those with yellow-orange color rims appear to owe at least part of their face-lifts to a form of lattice diffusion.
By the end of the meeting, the gathered masses had divided into two camps:
- Dealers who held stocks of such goods, along with gemologists who had issued heat-only reports on such goods
- Everyone else
Face-saving measures are now under discussion. Some have suggested the stones simply be referred to as “treated,” with a mention about their surface-based coloration. But as former AGTA President, Owen Bordelon stated at the Tucson meeting: “People are seriously deluded if they think these stones will fly off dealers’ shelves, even with such a description.”
Do we need new nomenclature for these stones? I don’t think so. Why should we invent a new name just because some stones were initially misidentified? Our current nomenclature will suffice.
According to Emmett, these new stones (those with well defined surface-conforming color rims), are in no way different than the blue lattice-diffused (Ti) stones of the past. With the blue sapphires, blue was diffused into colorless material as well as unevenly blue-colored material. Note that blue lattice diffusion also requires the presence of naturally-occurring iron in the stone to react with the inward diffusing titanium to produce the blue coloration. Given the similarities between the blue lattice diffusion of old and the orange lattice diffusion of today, logically the descriptions should be parallel. [Note: John Emmett has now reproduced these treatments in his lab – click this link for details]
 |
| Treated orange sapphires of the type described above, purchased in Bangkok by Pala International in Dec., 2001. Photo: Robert Weldon |
In solid-state physics, that which we gemologists formerly termed “surface diffusion” is referred to as “lattice” or “bulk” diffusion. This separates outside-in movement of light elements like hydrogen from similar outside-in movement of heavier elements like titanium, chromium and magnesium. It’s not a question of how deep the penetration, but more a question of what is going in.
The use of the term “lattice” (formerly ‘surface’) diffusion” in gemological nomenclature is an attempt to separate treatments that influence colorants already within a gem from those that introduce new colorants from outside. This relates to rarity, because treatments that depend on colorants already within the gem are limited in the changes they effect.
In contrast, treatments that involve outside-in movement of coloring agents (lattice diffusion) have far more leeway in the changes they can effect. Given a gem canvas that is relatively pure and light in color, treaters can theoretically paint color at will. This begs the question: when human intervention becomes such a large part of a gem’s apparent quality, why mess around? Why don’t treaters just get busy producing a full synthetic?
Deep down inside, we all know the answer to that one.
Prickly heat
Over the past twenty years, a number of controversies similar to this have occurred in our trade. In the late 1970’s and early 1980’s, it was the appearance of heat-treated rubies and sapphires (Hughes, 1995). Producers originally sold them as completely natural. When it became understood that the stones had been heated, they fought tooth and nail to avoid the disclosure of those treatments. Today disclosure is the norm.
In the early 1980’s, the first lattice-diffusion treated blue sapphires appeared. Producers initially sold them as natural, later as simply heated. Today, full disclosure is the norm.
The mid-1980’s saw Thai/Cambodian rubies with glass-filled surface cavities appear (Hughes, 1984). Again, initially sold as natural. Today, full disclosure is the norm.
By the late 1980’s, a second-wave of lattice-diffused stones appeared, with just a little color added on already blue stones with color zoning problems (Hughes, 1988, 1991, 1992). Producers initially denied the treatment, stating that stones had received only “surface heating.” The world’s labs did not accept this explanation. Today, disclosure is the norm and, in this particular case, such stones have largely disappeared from the market.[2]
In the early 1990’s, rubies from Möng Hsu appeared. Originally they were sold as simply heated. When glassy residues were found, producers stated this was just a byproduct of heating. It was later shown that such stones were deliberately heated in fluxes to heal their fractures with what amounts to synthetic ruby (Hughes and Galibert, 1998; Emmett, 1999; Hänni, 2001). Even today, some try to deny what is done to these stones, while many others do not fully understand it. But disclosure is becoming the norm.
Later in that decade, emerald oiling became an issue of controversy (Hughes, 1998, 2000). Producers and even some CIBJO members fought vigorously for over a decade to avoid disclosure. Today, disclosure is the norm.
Flash forward. 2002. Once again, we have a new treatment. And we are being told it involves one thing, while the evidence indicates another.
Déjà vu. Based on the historical record and current evidence, it appears only prudent to go slow with these stones.
Future games
A man’s eyes should be torn out if he can only see the past.
Russian proverb
In the end, attempting to equate a treated gem with one created by nature is a mistake. For far too long we in the colored stone business have tried to gloss over the difference. It is time we stopped trying and began admitting that there is a huge difference between a natural stone and an artificially enhanced product. If we value the natural product – if we wish it to survive in the marketplace – the only chance it stands is with full disclosure of all treatments.
Eight years ago I wrote the following:
Gem enhancements will not become any less effective, nor will detection become easier. Such a clever cat, the trade asked for a better mousetrap, but now complains because all the mice are dead and it has nothing to eat.
We used to believe in magic. We thought that everyone could get rich by making silk purses out of sows’ ears. But we failed to see into the future. We rubbed the magic lamp, the enhancement genie appeared, but now he’s turned on his master. And suddenly we’ve decided that we don’t believe in magic after all.
I still believe in magic. I still remember the magic that holding a fine Burma ruby first brought. Today my daughter is five years old. I hope that when she is my age, she still believes in magic. I hope that when she holds a fine gem, she holds a silk purse, not a sow’s ear.
Maybe it’s that time again – time to look ahead to what the future might hold – and time to prepare for that future.
![]()
References. A detailed description of heat treatment in corundum can be found in the following references:
- Emmett, John L. and Douthit, Troy R. (1993) Heat treating the sapphires of Rock Creek, Montana. Gems & Gemology, Vol. 29, No. 4, pp. 250–272.
- Emmett, John L. (1999) Fluxes and the heat treatment of ruby and sapphire. Gems & Gemology, Vol. 35, No. 3, pp. 90–92.
- Hänni, Henry A. (2001) Beobachtungen an hitzegehandeltem Rubin mit künstlicher Rissheilung (Observations on heat-treated ruby with artificially healed fissures). Zeitschrift der Deutschen Gemmologischen Gesellschaft, Vol. 50, No. 3, pp. 123–136.
- Hughes, R.W. (1984) Surface repaired rubies – a new gem treatment. Jewellery News Asia.
- Hughes, R.W. (1988) Reappearance of surface-diffusion treated sapphires in Bangkok. ICA Lab Alert, No. 12, 2 pp.
- Hughes, R.W. (1991) There’s a rumble in the jungle – The sapphire face-lift face-off saga. Gemological Digest, Vol. 3, No. 2, pp. 17–31.
- Hughes, R.W. (1992) Devil’s Advocate: Vampire blues: deep-diffusion treated sapphires. JewelSiam, No. 3, May-June, pp. 83–86.
- Hughes, R.W. (1995) A brief history of heat. Australian Gemmologist, Vol. 19, No. 2, pp. 52–54.
- Hughes, R.W. (1997) Ruby & Sapphire. RWH Publishing, Boulder, CO, 512 pp.
- Hughes, R.W. (1998) Cloak and dagger: The politics of Opticon. GQ Eye, Vol. 1, No. 1, Fall, pp. 3, 6.
- Hughes, R.W. (2000) The Digital Devil: Check Your Gut Instincts at the Door. GemKey Magazine, Vol. 2, No. 3, March–April, pp. 62–63, 104.
- Hughes, R.W. and Galibert, O. (1998) Foreign affairs: Fracture healing/filling of Möng Hsu ruby. Australian Gemmologist, Vol. 20, No. 2, April–June, pp. 70–74.
- Mohapatra, S.K. and Kröger, F.A. (1977) Defect structure of alpha-Al2O3 doped with magnesium. Journal of the American Ceramic Society, Vol. 60, No. 3–4, pp. 141–148.
- Schmetzer, K., Bosshart, G., Hänni, H. (1983) Naturally-colored and treated yellow and orange-brown sapphires. Journal of Gemmology, Vol. 18, No. 7, July, pp. 607–622.
About the author. Richard Hughes is the author of the classic Ruby & Sapphire. He can be contacted at: rubydick@ruby-sapphire.com, or through his personal web site, Ruby-Sapphire.com.
Acknowledgments. This article could not have been written without the generous help of the following (in alphabetical order): Jeff Bilgore, Edward Boehm, Kriengkrai Chiaraput, Terry Coldham, Richard Drucker, John Emmett, Josh Hall, John Koivula, William Larson, Gabrièl Mattice, Shane McClure, Gene Meieran, Yianni Melas, Roland Naftule, Karen Palmer, Visut Pisutha-Arnond, Stuart Robertson, Gary Roskin, Ken Scarratt, James Shigley, Arnold Silverberg, Mark Smith, Maha Tannous, Vichian Veerasaksri, Pornsawat Wathanakul, Robert Weldon, Ray Zajicek and Urs Zwyssig.
Afterword. This article will be appearing in the next issue of The Guide (2002, Vol. 21, No. 2, March–April). This web edition contains material and updates not found in the print version.
Note. The title illustration is a gem stylistically altered by the author in Photoshop. It is not one of the treated orange sapphires discussed in the article.
| Notes on Diffusion in Corundum |
For the following description of diffusion in corundum, we thank John Emmett of Crystal Chemistry, Brush Prairie, WA. But please note that the account should be thought of as preliminary only, since Mr. Emmett has not written this himself. In other words, all mistakes are mine, not those of Mr. Emmett. Over the years, a number of claims have been made regarding heat treatment. Perhaps the most common is that “nothing is added” during heat treatment. Current scientific evidence simply does not support this idea. During the heat treatment of corundum, elements and subatomic particles shift both state and position, including atoms moving into the gem from outside. Alteration of the valence states of impurity atoms is particularly important. The latter is done via diffusion (movement) of hydrogen (H) or aluminum (Al) vacancies in or out of a stone. Even though it might seem like nothing, hydrogen is a chemical. You find it on the periodic table of the elements, just like titanium (Ti), chromium (Cr), nickel (Ni) and vanadium (V). Corundum is made up of Al2O3, with the aluminum in the Al3+ valence state. Impurity elements with the same valence, such as Cr3+, Fe3+ and V3+ will easily and happily substitute for Al3+ in the corundum structure. These elements require substitutions in the 0.1–3% range to produce significant color in corundum. Another type of coloration, however, is caused by color centers, and it requires far smaller amounts of impurities. In these cases, allovalent ions such as Mg2+ and Ti4+ are involved. When an allovalent ion replaces Al3+, the fit is not so happy, and defects are created which absorb light. The most important situation is where lower valence ions (such as Mg2+) are present in larger quantities than higher valence ions (Ti4+). When this requirement is met, yellow-producing trapped-hole color centers can result. Now we come to the good part. Heating a gem in the presence of oxygen can activate such color centers in corundum. Virtually any light-colored Sri Lankan sapphire can be heated to produce a yellow to golden color in an oxidizing atmosphere because these stones naturally possess the Mg2+ needed. In the case of Montana sapphires (Rock Creek, Missouri River), the Mg2+ is often present in greater concentrations in the core of the crystal. Thus heating in an oxidizing atmosphere causes a deep yellow core in many stones. Heating a pink stone may add the yellow-producing color centers to the pink color, causing a padparadscha-like color, if the above conditions are met. Heating a purple stone adds yellow to the purple, thus producing a redder, more ruby-like stone. Heating a blue stone adds yellow to the blue, which is not normally done because it muddies the blue. But what if your stone does not have the needed allovalent ion? You can diffuse it in from the outside. While diffusion rates for Cr3+, Fe3+ and V3+ are agonizingly slow, allovalent elements such as Mg2+ and Ti4+ diffuse into corundum some 10,000 times faster. Thus it is possible to add significant color to corundum, via diffusion of tiny quantities of allovalent ions from outside. How much is needed? It may be as little as a few tens of parts per million. Such traces are virtually undetectable with the types of instruments found in even the most well equipped gemological labs. In some cases, burners may be diffusing allovalent ions into their stones without even realizing it. Indeed some fluxes commonly used in burning (such as borax) may contain traces of Mg2+. Other allovalent ions that can possibly produce this effect include lithium, beryllium and sodium. Dr. Emmett has reproduced these diffusion experiments in his laboratory. In some cases, he found that traces of Mg2+ contamination enough to produce some coloration were found even in the highly purified alumina from the crucibles. Post-burn yellow or cream coloration of a previously snow-white alumina crucible may be evidence of such contamination. When Ti4+ is diffused into corundum to produce a blue color, it moves in quickly until the point where it meets Fe ions. At that point, bonds are formed with Fe and the diffusion generally ceases. This is why we often find a sharp blue boundary between a diffused zone and the colorless core in lattice-diffusion treated blue sapphires. Mr. Emmett has done controlled burns of Ti lattice diffusion with sapphires of varying Fe contents that confirm this effect. Penetration of blue color is more diffuse in stones of lower Fe content. With stones of higher Fe content, a sharper boundary is found between the blue diffusion skin and the crystal core. With Mg2+, however, no such bonding with iron occurs. The result is that Mg2+ diffusion into corundum does not produce the same sudden color transitions and darkened facet junctions as those found with blue Ti4+-based diffusion. All this theory goes a long way towards explaining the types of things we have been seeing over the past few weeks with these new orange sapphire treatments. Burners may be burning stones with nothing other than oxygen and if a stone naturally possesses enough of the proper impurity, it may develop an orange color. In some stones, where the impurity is present only in one area, just that area will turn yellow (or orange). With other stones, the impurity may be diffused in from outside, via a flux or other impurity present during the burn. And in other cases, we may see a combination effect, where some impurity is diffusing in from outside, along with an activation of impurities already present in the crystal. Further details of the diffusion process can be found in Chapter 6 of my book, Ruby & Sapphire. Much of that material is based on discussions with John Emmett. Thus I would like to again thank him for his help.
Possible scenario for blue (Ti) and yellow (Be) lattice (bulk) diffusion. The yellow lattice diffusion is over a pink stone, creating an orange (padparadscha) color. Note that the separation between the Ti diffusion and the core color tends to be sharp, while that between the Be diffusion and the core color is far less distinct. Ti diffusion produces a dark girdle and facet junctions, while the Be diffusion does not. In some cases, a stone may naturally possess impurities that can be activated by the treatment. Thus you may have yellow-orange areas completely within a gem, where the impurities were not diffused in from outside. Combination effects are also possible (see illustration at right). Illustration © R.W. Hughes |
| The Diffusion Mechanism |
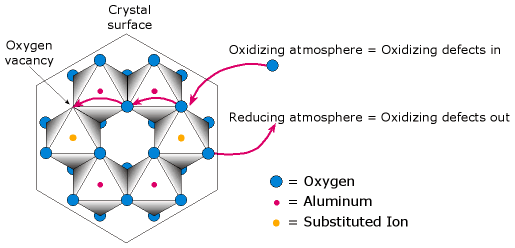
One method by which heat treatment produces changes in color is via diffusion. For diffusion to occur, lattice defects are required; the best way of creating such defects is via heating, as defect percentages increase with temperature. Lattice defects allow the movement, or diffusion, of impurity atoms through the gem. Diffusion of oxidizing defects into corundum (oxidizing atmosphere) can change Fe2+ to Fe3+, while diffusion of oxidizing defects out of the gem (reducing atmosphere) changes Fe3+ to Fe2+. This can affect color. However, in most corundums, reduction of Fe3+ to Fe2+ has a problem, in that, in iron-rich stones, the iron unmixes in the form of iron spinel (hercynite; Fe2+Al2O4). Ti4+ seems to assist the reduction, combining with the Fe2+ in pairs that replace a pair of Al3+ atoms. Coloring agents themselves (such as Fe, Ti, Cr, V and Mg) may also be introduced into the stone (a process known as lattice or bulk diffusion), but penetration is generally limited to the gem’s surface regions because diffusion rates for such elements are quite slow. Diffusion rates vary according to the elements involved and their valence. Allovalent titanium (Ti4+), magnesium (Mg2+), lithium (Li1+), beryllium (Be2+), calcium (Ca2+) or sodium (Na1+) diffuse into corundum some 10,000 times faster than either Fe3+ or Cr3+ because replacement of Al3+ by an allovalent impurity stimulates formation of defects (John Emmett, pers. comm., 27 June, 1994; 15 Jan., 2002).
The above illustration shows a view of the corundum atomic structure looking parallel to the c-axis. Oxygen does not really move throughout a gem. Instead, through a chain reaction, the movement of point defects (vacancies) allows free oxygen from the atmosphere to produce changes even deep within a gem. Changing the furnace atmosphere produces a net gain or loss of oxygen, which can affect the valence state of iron, thus influencing color. Illustration © R.W. Hughes |
_______________
[1] While John Emmett is not one to blow his own horn, allow me to elaborate a bit on his background. From 1975–1988, John was Associate Director for Lasers at Lawrence Livermore National Laboratory in Livermore, CA. It was here that he first began researching corundum, something that continues to this day with his own company, Crystal Chemistry, Brush Prairie, WA. While at Lawrence Livermore, the programs involved over 1500 researchers, including 300 Ph.D.’s, and in 1988 alone were funded at US$250 million. He has authored over 50 papers published in peer-reviewed scientific journals. John is considered a world authority on the physics and chemistry of corundum and has for years been involved in heat treatment.
[2] Such stones have largely disappeared because the market decided it would pay more for a heated sapphire with zoning problems than a lattice-diffusion heated sapphire with no zoning.